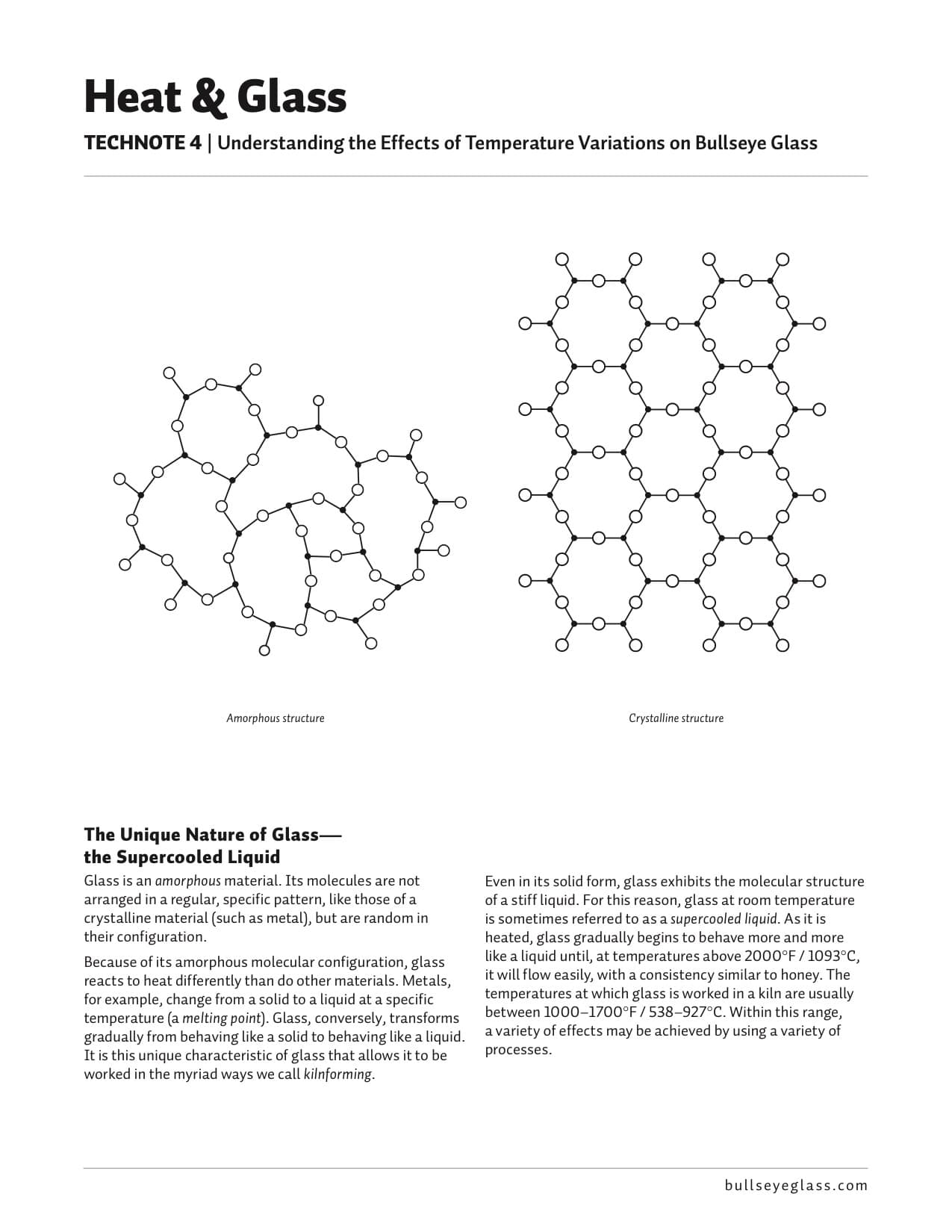
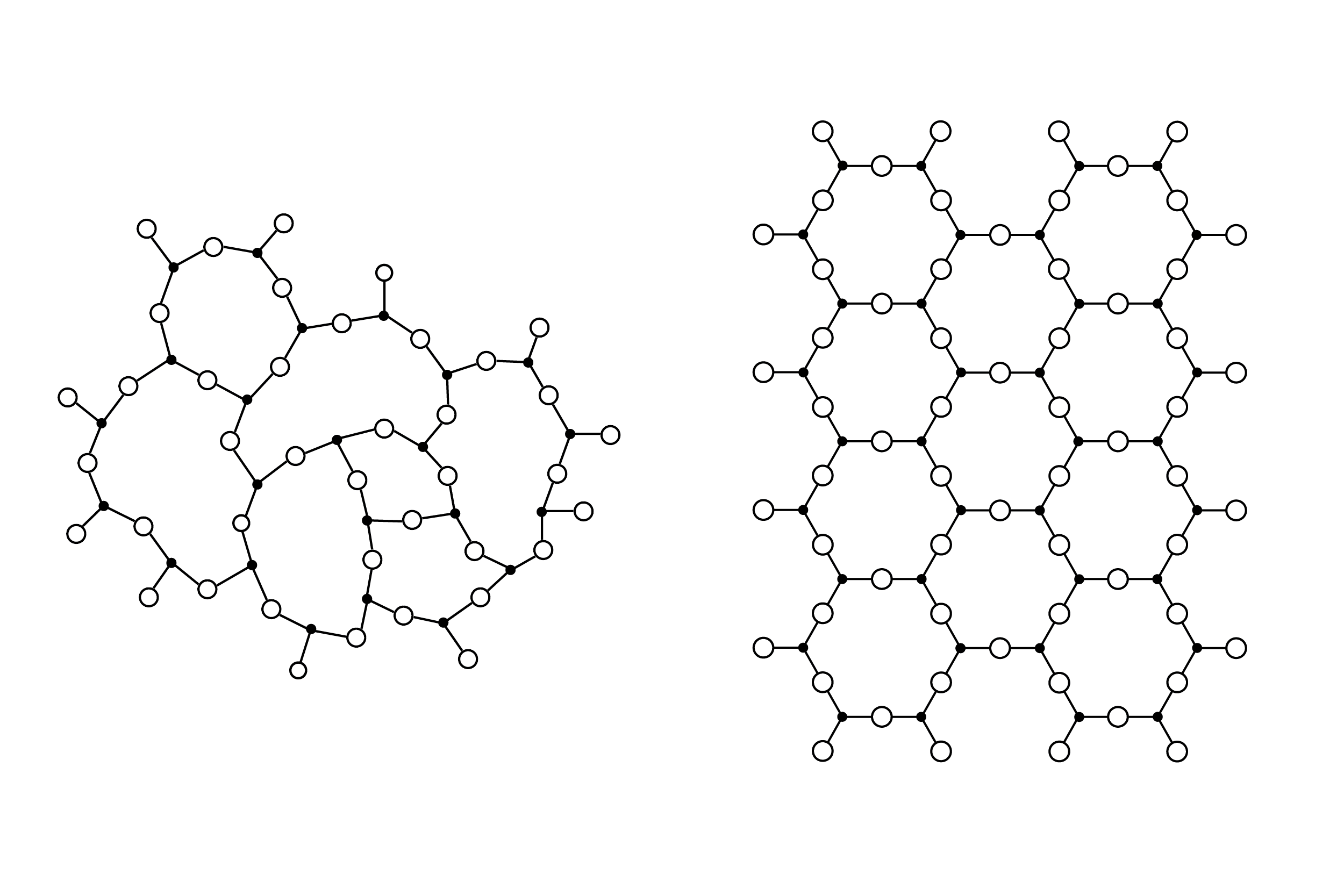
The Unique Nature of Glass—the Supercooled Liquid
Glass is an amorphous material. Its molecules are not arranged in a regular, specific pattern, like those of a crystalline material (such as metal), but are random in their configuration.
Because of its amorphous molecular configuration, glass reacts to heat differently than do other materials. Metals, for example, change from a solid to a liquid at a specific temperature (a melting point). Glass, conversely, transforms gradually from behaving like a solid to behaving like a liquid. It is this unique characteristic of glass that allows it to be worked in the myriad ways we call kilnforming.
Amorphous structure Even in its solid form, glass exhibits the molecular structure of a stiff liquid. For this reason, glass at room temperature is sometimes referred to as a supercooled liquid. As it is heated, glass gradually begins to behave more and more like a liquid until, at temperatures above 2000°F / 1093°C, it will flow easily, with a consistency similar to honey. The temperatures at which glass is worked in a kiln are usually between 1000–1700°F / 538–927°C. Within this range, a variety of effects may be achieved by using a variety of processes.